
COVID-19 Vaccines Advice
Why get vaccinated against COVID-19?
The emergency phase of COVID-19 is over, but the virus continues to spread widely across the globe and endanger people's lives, particularly those who are older, have chronic diseases, are immunocompromised or pregnant.
Safe and effective vaccines help ensure that COVID-19 does not result in severe disease and death. In 2021 alone, COVID-19 vaccines saved at least an estimated 14.4 million lives worldwide.
Check your COVID-19 vaccination status and consult with your healthcare provider for any needed doses.
COVID-19 vaccines are safe.
Strict precautions are in place to help ensure the safety of all COVID-19 vaccines.
Before receiving validation from WHO and national regulatory agencies, COVID-19 vaccines were subject to rigorous testing in clinical trials to prove that they meet internationally agreed benchmarks for safety and efficacy.
Unprecedented scientific collaborations, extensive prior research and substantial public funding enabled swift COVID-19 vaccine development to be completed in record time - while maintaining high safety standards. New versions of the vaccine are being developed as the COVID-19 virus continues to circulate and change.
Since 2021, more than 13 billion COVID-19 vaccine doses have been administered globally. As with all vaccines, WHO and regulatory authorities continuously monitor the use of COVID-19 vaccines to identify and respond to any safety issues that might arise. Serious reactions to COVID-19 vaccines are extremely rare. Through this process, we establish that COVID-19 vaccines remain safe worldwide.
Read more on the safety of COVID-19 vaccines:
- Questions and Answers: Coronavirus disease (COVID-19): Vaccine safety
Who should get vaccinated?
In September 2024, WHO's Strategic Advisory Group on Immunization (SAGE) reaffirmed the validity of its recommendations on COVID-19 vaccination and the importance of revaccination for groups at higher risk of severe disease and death (older adults, people iwht comorbidities, immunocompromised individuals and pregnant persons). Revaccination of health workers is also recommended.
The table below outlines the updated recommendations based on a person’s (1) vaccination history and (2) age and health condition. 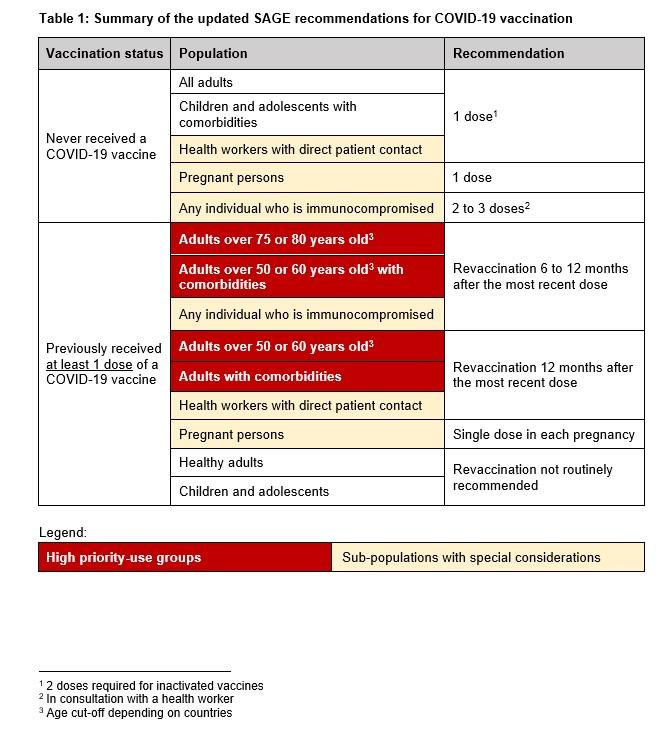
Getting vaccinated: Specific advice
- Take whatever vaccine is made available to you, even if you have already had COVID-19. Hybrid immunity (protection from both vaccination and COVID-19 infection) enhances protection against severe disease of future COVID-19 infections and confers longer protection.
Revaccination approximately 12 months after the previous dose is particularly important for those groups at higher risk of severe disease and mortality (older adults, people with chronic diseases, pregnant persons, immunocompromised persons) and health workers.
- If you are pregnant, want to get pregnant in the future or are currently breastfeeding, getting vaccinated is important to protect you and your family. Many people around the world have now been vaccinated against COVID-19 while pregnant or breastfeeding, and no safety concerns have been identified for them or their babies.
A single dose during pregnancy is recommended, regardless of prior vaccination. Read more: COVID-19 Q&A: Pregnancy, childbirth and the postnatal period.
It is safe and effective to receive different COVID-19 vaccines and to co-administer other vaccines during the same visit; for example, with other vaccines recommended during pregnancy such as the seasonal influenza vaccine.
- You should not be vaccinated if:
- You have a history of severe allergic reactions/anaphylaxis to any of the ingredients of the COVID-19 vaccine.
- You have a fever over 38.5ºC on the day of your vaccine appointment. Postpone until you have recovered.
- If you are on blood thinners, it is safe for you to get vaccinated, but let the person vaccinating know.
What to expect after getting vaccinated
- Some people will experience mild side effects after being vaccinated against COVID-19. Common side effects of COVID-19 vaccines include fever, head or body aches and a sore arm. These symptoms usually go away within a day or two and, if needed, can be managed by getting some rest, drinking fluids, or taking pain-relieving medications.
Contact your healthcare provider if you are worried about any of the side effects that you are experiencing.
If you do get COVID-19 after vaccination, you are more likely to have mild or no symptoms than if you hadn’t been vaccinated.
- Vaccination should not be delayed in anticipation of newer versions of the COVID-19 vaccine. Currently approved COVID-19 vaccines, including those based on the index virus and the bivalent vaccines, continue to protect against severe disease.
For people at a high risk of getting severe COVID-19, a dose of any available vaccine is more beneficial than delaying vaccination.
Many COVID-19 manufacturers have developed or are in the process of developing updated vaccines to target the current circulating variants. When such vaccines become available, those eligible for revaccination could consider such vaccines.
- It takes a couple of weeks after each dose of the COVID-19 vaccine for your body to develop maximum levels of immunity - you are not protected right away. Protection against COVID-19 severe disease and death is highest in the first few months after vaccination and then starts to decrease. The duration of protection can vary depending on vaccination history, past infection, the type of vaccine received and circulating COVID-19 variants.
- It is still possible to get COVID-19 and spread it to others after being vaccinated, so continue to do everything you can to keep yourself and others healthy.Wash your hands regularly and cover coughs and sneezes and follow the advice of your health authorities.
Link nội dung: https://giaitri.edu.vn/vacvin-a72814.html