What is Silicon dioxide?
SiO2 is an oxide of silicon with a chemical name silicon dioxide. It is also called Silica or Kalii bromidum or Silicic oxide or silicic acid. It is widely found in nature as quartz.
It is obtained as a transparent to grey, in its crystalline or amorphous powdered form. It is odourless and tasteless compound.
Table of Contents
- Recommended Videos
- Properties of Silicon dioxide
- Silicon dioxide Structure
- SiO2 Uses
- Production of Silicon dioxide
- Silicon dioxide Reactions
- Health hazards
- Frequently Asked Questions - FAQs
Recommended Videos
Properties of Silicon dioxide - SiO2
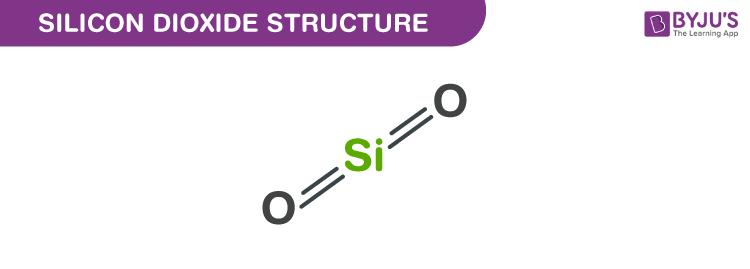
SiO2 Silicon dioxide Molecular weight of SiO2 60.08 g/mol Density of Silicon dioxide 2.648 g/cm3 Melting point of Silicon dioxide 1,713 °C Boiling point of Silicon dioxide 2,950 °CSilicon dioxide Structure - SiO2
SiO2Uses (Silicon dioxide)
- Silicon dioxide is used in the construction industry to produce concrete.
- In its crystalline form it is used in hydraulic fracturing.
- Used in the production of glass.
- Used as a Sedative.
- Used in the production of elemental silicon.
- Used as an anti-caking agent in powdered foods like spices.
- Used as a fining agent in juice, beer, and wine.
- Used pharmaceuticals for making tablets.
- Used in toothpaste to remove tooth plaque.
Production of Silicon dioxide
Amorphous silica or precipitated silica is obtained by the acidification of sodium silicate solutions. Silica gel is washed and dehydrated to produce colourless microporous silica. The reaction involving a trisilicate along with sulphuric acid is given below:
Na2Si3O7 + H2SO4 → 3SiO2 + Na2SO4 + H2O
Silicon dioxide Reactions
- Silica gets converted to silicon by reducing with carbon.
- Fluorine when reacted with silicon dioxide it produces SiF4 and O2.
- Silicon dioxide reacts with hydrofluoric acid to produce hexafluorosilicic acid (H2SiF6).
SiO2 + 6HF → H2SiF6 + 2H2O
- In silicon dioxide, each Si atom is surrounded by 4 oxygen atom and each oxygen atom is bonded to 2 silicon atom.
Health hazards
Silica when ingested orally is non-toxic. As per a study conducted in the year 2008, found that the higher the levels of silica in water, the risk of dementia decreased. Therefore, the dose was increased to 10 mg/day of silica in drinking water as the risk of dementia decreased. When finely divided crystalline silica dust is inhaled, it can lead to bronchitis, lung cancer, or silicosis, due to the lodging of dust in the lungs. When fine silica particles are inhaled in large enough quantities, it increases the risk of rheumatoid arthritis and lupus.
Learn more about the Structure, physical and chemical properties of SiO2 from the experts at BYJU’S.


